You know your consumer product is scaling when Katie Couric gives it a shout out. It’s even better when the product relates to something Ms. Couric feels very strongly about – colon cancer. Her husband died of colon cancer at the age of 42 in 1998. Several years later, Ms. Couric had a live colonoscopy performed on the “Today” show which sparked “a surge in colon cancer screenings doctors nicknamed the Katie Couric effect.” That’s according to an article by Fierce Biotech that talks about the timely relationship between Exact Sciences (EXAS) and Katie Couric Media.
The campaign comes as the U.S. Preventive Services Task Force last May lowered the recommended colorectal cancer screening age from 50 to 45. The change makes an additional 45 million Americans eligible for screening—and opens up a bigger potential market for Cologuard.
Credit: Exact Sciences
While the math doesn’t seem to add up according to age category population numbers (it’s more like 20 million Americans aged 45 to 49) it’s still a big number. If half that population tested annually at a cost of $500 per test, that’s a recurring revenue stream of $5 billion that’s waiting to be captured. However, the American Cancer Society recommends a screening interval every 3 years with Cologuard following a negative result, so the $5 billion every three years represents a $1.66 billion annual run rate.
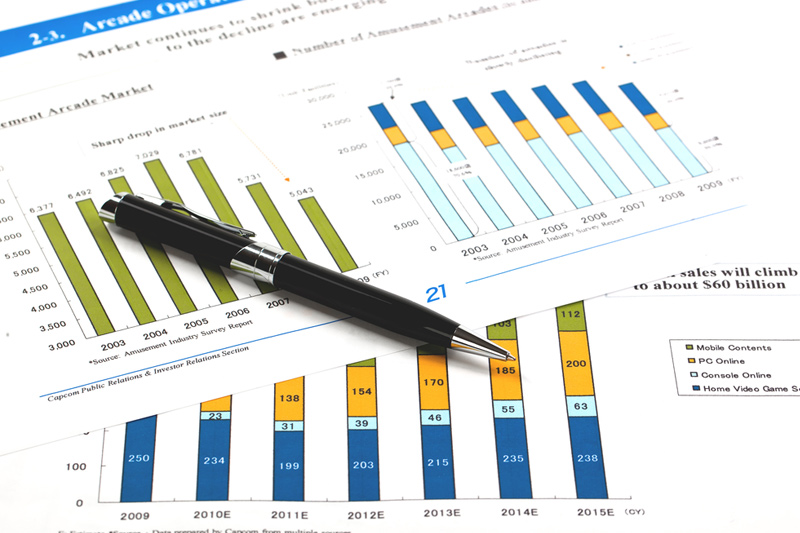
Exact Sciences is engaged in a giant push towards getting more Americans to screen for colon cancer as part of routine checkups. While everyone runs around raising funds to “cure cancer,” they should be focused on early screenings. The odds of survival skyrocket when cancer is detected early across all types. The yellow bars below show the percentage of people living past five years when cancer is caught early vs the purple bars which is the same for advanced cancer (metastatic).
Regular colorectal screenings become a recurring revenue stream – a software ass a service business model if you will – which will bring in an expected $1.3 billion of screenings revenue for Exact Sciences in 2022. In our last piece on the company back in 2018, we noted a $5.5 billion potential opportunity before the market expanded. From the Exact Sciences 10-K:
If the test were used by 40-percent of the 85 million people that we estimate to be eligible for screening in the U.S. between the ages of 50-85, at a three-year interval, and if average revenue per test was $500, we estimate that our annual Cologuard revenue would be more than $5.5 billion.
Credit: Exact Sciences
You’re probably getting peeved because we haven’t even mentioned Guardant Health (GH) yet. Patience young grasshopper. It’s important to understand what Exact Sciences is up to because they’re closely related to a company whose share price fell 30% today – Guardant Sciences. It’s no coincidence that Exact Sciences is up +20% on the same news. Whatever news just came out from Guardant has convinced investors that Exact Sciences’ competition just got weaker.
Setting Expectations
The short story is that Guardant published results from a massive study they’ve been working on for their colorectal cancer blood test. Management thought it was groundbreaking stuff, while the investment community sold off shares. Bloomberg published a piece which offers up no insights as to why the drop happened, while Guardant quickly contacted them with the below statement:
“There’s some lack of understanding of the fact that this is strong data,” Talasaz said. “It’s the data that’s going to pave the path for a FDA approval.”
Credit: Bloomberg
Guardant’s management team is trying to spin the news as a win, but we’ll take what the market says over company management any day. What could the market have seen in yesterday’s press release that was concerning? Here are some bits and bobs we might explore along with a visual depiction of how sensitivity and specificity differ.
The test demonstrated 83% sensitivity for the detection of colorectal cancer with specificity of 90%. Medicare provides coverage for sensitivity greater than or equal to 74% and specificity greater than or equal to 90%.Among the initial 8,000 individuals for whom the test was ordered during a routine visit with their physician, 90% completed the test. This is in stark contrast with adherence rates ranging from 43% to 66% for other non-invasive stool tests.
Perhaps that first bullet point is concerning because the specificity result is the minimum required which may raise some eyebrows come time for the Medicare coverage decision? Guardant’s emphasis on a higher adherence in bullet point two is clearly a nod to regulators who they hope to woo when submitting an FDA approval application in early 2023. To figure out just what investors were thinking, we listened to the conference call that accompanied the results which had everyone talking about two key numbers highlighted in yellow below.
CRC stands for colorectal cancer, so CRC sensitivity is the ability of the test to identify colorectal cancer in patients. AA stands for advanced adenoma, and it’s not as important as you might think (so sayeth Guardant). Both numbers highlighted in yellow above are what seem to have caused the dramatic drop in share price.
A Momentous Occasion
Management tried to pass it off as a momentous occasion, but Puneet from SVB Securities wasn’t having it, and came out of the gate swinging. Why is the 83% CRC sensitivity significantly below the high 80s and low 90s that they were seeing throughout data sets leading up to these final results? Why was this degradation not part of the company’s expectations from the start, especially given that other trials should have given them every indication this was going to happen?
The response didn’t answer the questions, but asked the analyst to consider current options, what sensitivities these tests were being provided at. In other words, it’s better than nothing. This is a starting point, we may get better. The next question by Puneet focused on the advanced adenoma 13% number which came in lower than the 20% that was being thrown about before. Can Guardant even use that indication on the FDA label when the number is that low? More on this in a bit.
Guardant believes as long as they’re above 76% or 77% on CRC sensitivity, they’re in a domain to get approved. They commented on the 68-69% sensitivity of the Epi proColon test, the only blood-based biomarker test for colorectal cancer screening that was approved by the FDA in 2016. Another analyst brought up the impact of the results on test pricing, and management didn’t think this would affect that variable.
The usual “great results guys” group wank wasn’t observed during this call as analysts lobbed challenging questions around the reported results which didn’t seem to match their expectations. One analyst chided the sharp drop in share price and suggested a Nobel Prize was in order, and he sounded absolutely smashed when he said it. Guardant responded by saying that the 13% might be a big factor for the drop. They then pointed out that only about 5% of AAs become cancer, and usually it takes about 10 years for them to mature into the big “C.”
Management continued to reiterate how happy they were with the results much to the chagrin of those who felt otherwise, and listening to the one-hour call was actually more informative than we thought. You can listen to it here.
Our Take
Analysts who follow Guardant typically have complex business models they use to perform sensitivity analyses using every variable you can think of, one being the detailed results of a large test conducted with 8,000 patients that provides the foundation for FDA approval. When the results didn’t match their expectations, the models adjust valuations, and that’s when selloffs happen. Guardant seems to think these results will be sufficient for FDA approval, so that’s good enough for us. Will a test adoption ultimately be affected by these lower numbers? Seems like that all comes down to marketing.
Critics of Cologuard have claimed that more closely examining the details show a test that’s less than optimal. The Truth About Cologuard Tests is an article that claims colonoscopies are the only suitable method to find polyps. The same holds true for cancer blood tests as well, but having a celebrity advertise your product means the sheep will start using it regardless of the nitty-gritty details.
One analyst asked Guardant about how the results impacted targets to achieve breakeven targets – 83% vs 85% sensitivity. The answer was that these data points are in the same zip code, and that based on what they’re hearing from customers, this data allows them to hit all their targets. Looking to the future, Guardant believes that being able to test for multiple types of cancer in a single blood test will help them displace stool-based tests. That means all eyes on their ongoing lung cancer test.
If/when FDA approval happens, Guardant needs to start selling their test in large quantities to capture market share as quickly as possible. Our recent piece on Freenome talked about just how competitive this space is. Management believes that get to 10 million annual tests in ten years. As long as they get FDA approval, they believe that 83 or 85 or 86 sensitivity just doesn’t change the overall results in a meaningful way.
Conclusion
The price action affecting both companies means investors have perceived the results from Guardant as providing Exact Sciences with some competitive advantage. From the questions posed on their call, it appears that key metrics around sensitivity and AA detection didn’t meet analysts’ expectations. If the company believes they’ll get FDA approval with these numbers, then that event will provoke volatility in the opposite direction.
The drop seems to be around short-term metrics which can potentially improve over time. Guardant talks about their large database of data gathered can be now used to improve their test performance. It’s the beginning of the story, not the end. Let’s hope so.
Tech investing is extremely risky. Minimize your risk with our stock research, investment tools, and portfolios, and find out which tech stocks you should avoid. Become a Nanalyze Premium member and find out today!